Rapisure COVID-19 at Home Antigen Test

FDA-EUA Authorized 1-3 COVID-19 Antigen Test for OTC and Point of Care
The Rapisure COVID-19 At Home Antigen Test is a lateral flow immunoassay device intended for the qualitative detection of nucleocapsid protein antigens from SARS-CoV-2.
- Made in the USA
- Simple, Quick, Accurate
- Rapid Result in 10 minutes
For FDA Emergency Use Authorization (EUA) only. For In Vitro Diagnostic use.
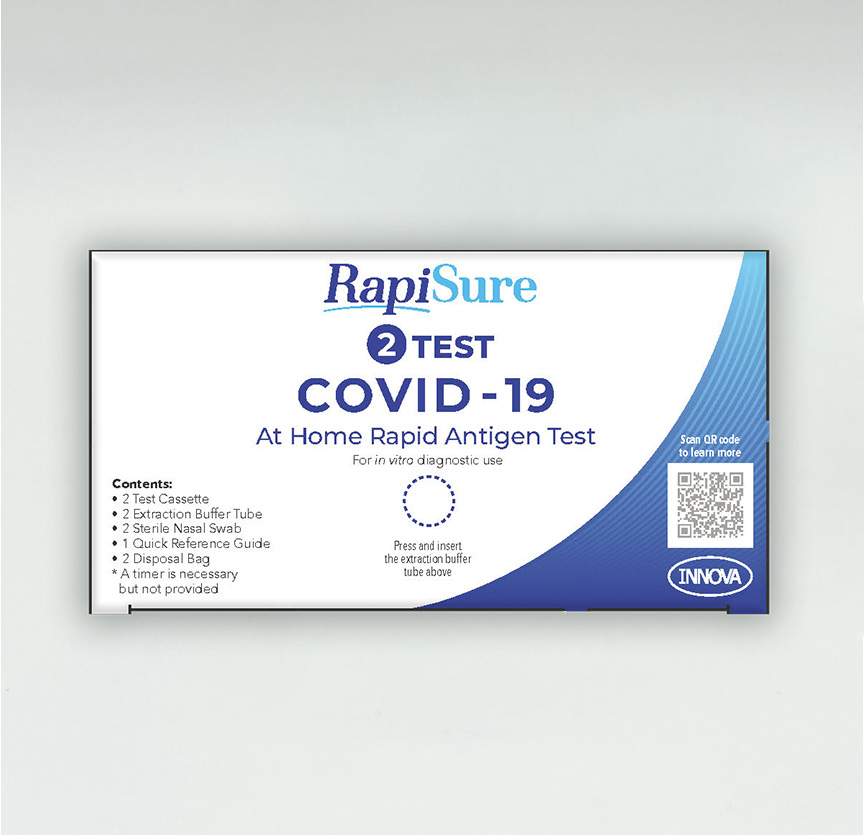
Kit Information
Contents of Each Test Kit:
- Disposable Nasal Swab
- Extraction Buffer Tube
- Test Cassette
- Disposable Bag
- Quick Reference Guide
Packaging
Test kit is available in:
- 1 Test
- 2 Tests
- 5 Tests
- 25 Tests
Specifications
- Sample type: Nasal swab
- Accuracy: 98%
- Time to result: 10 minutes
How It Works
Test Procedure
For more information of all the test procedures, please download the Quick Reference Guide (QRG) at the bottom of this page.
Prepare
Remove nasal swab from its packaging. DO NOT touch the swab tip
Swab
Gently insert swab ½ - ¾ inch into first nostril, or until you feel resistance. Slowly make at least 5 rotations with the swab firmly against the walls of the nostril for approximately 15 seconds. Repeat step in your second nostril using the same swab.
Stir & Squeeze
Take the tube out of the tube holder. Place the swab into the tube, ensure the swab tip is in the liquid inside the tube. Stir the swab tip against the bottom and side of the tube for at least 15 times. While squeezing the sides of the vial firmly, pull the swab out to remove excess liquid.
Drip
Add 3 drops of solution into the circular sample well, labeled as "S" on the test cassette.
Wait 10 minutes
Set timer for 10 minutes. Do not move or lift the test cassette. Read the test result at 10 minutes.
Advin FAQs
Ordering Information
| Photos | # Units | Catalogue Number | Model Number | Price | |
|---|---|---|---|---|---|
| . | . | . | . | . | . |
| . | . | . | . | . | |
| . | . | . | . | . | . |
FAQ's
Potential risks include:
- Possible discomfort during sample collection.
- Possible incorrect test result (see Warnings and Result Interpretation sections for more information).
Potential benefits include:
- The results, along with other information, can help you and your healthcare provider make informed recommendations about your care.
- The results of this test may help limit the potential spread of COVID-19 to your family and others in your community.
For more information on EUAs go here:
There are different kinds of tests for the SARS-CoV-2 virus that causes COVID-19. Molecular tests detect genetic material from the virus. Antigen tests, such as the Advin COVID-19 Antigen Test @Home, detect proteins from the virus. Due to the lower sensitivity of antigen tests, there is a higher chance this test will give you a false negative result when you have COVID-19 than a molecular test would.
Clinical studies have shown that antigen tests more accurately determine whether you are infected with the virus that causes COVID-19 when taken multiple times across several days. Repeat testing improves test accuracy. This serial testing approach is recommended to minimize the risk of incorrect results. For more information on the performance of the test and how the performance may apply to you, please refer to the performance data in the Healthcare Provider Instructions for Use (IFU), available at http://www.advinbio.com.
A positive result means that it is very likely you have COVID-19 because proteins from the virus that causes COVID-19 were found in your sample. You should self-isolate from others and contact a healthcare provider for medical advice about your positive result.
A negative test result indicates that antigens from the virus that causes COVID-19 were not detected in your sample. However, if you have symptoms of COVID-19, and your first test is negative, you should test again in 48 hours since antigen tests are not as sensitive as molecular tests. If you do not have symptoms and received a negative result, you should test at least two more times with 48 hours in between tests for a total of three tests. If you have a negative result, it does not rule out SARS-CoV-2 infection; you may still be infected and you may still infect others. It is important that you work with your healthcare provider to help you understand the next steps you should take.
An invalid result means the test was not able to tell if you have COVID-19 or not. If the test is invalid, a new swab should be used to collect a new nasal specimen and you should test again with a new test.
IMPORTANT
Do not use this test as the only guide to manage your illness. Consult your healthcare provider if your symptoms persist or become more severe. Individuals should provide all results obtained with this product to their healthcare provider.
Learn More
For more information about our products or to make a purchase, please contact us using the form and we will get back to you shortly.
Disclaimers
- This product has not been FDA cleared or approved, but has been authorized by FDA under an EUA;
- This product has been authorized only for the detection of proteins from SARS-CoV-2, not for any other viruses or pathogens; and,
- The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb- 3(b)(1), unless the declaration is terminated or authorization is revoked sooner.
- Determining a negative result requires multiple tests. You may need to purchase additional tests to perform serial (repeat) testing. This test is more likely to give you a false negative result when you have COVID-19 than a lab-based molecular test.
References
- COVID-19 Fact Sheet for Healthcare Professionals
- Rapisure COVID-19 At Home Antigen Test Quick Reference Guide
- Healthcare Provider Instructions for Use (IFU)
