QMC HealthID™
Innova Medical Group

The app enables organizations to control and monitor the health status information of their stakeholders.
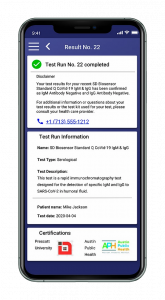
 The QMC HealthID™ Smartphone App tracks and reports test results privately with encrypted blockchain security. It is adaptable to various healthcare partners with its open architecture. HIPAA compliant, this easy-to-use app allows a person’s health status to be authenticated without sharing privacy data.
The QMC HealthID™ Smartphone App tracks and reports test results privately with encrypted blockchain security. It is adaptable to various healthcare partners with its open architecture. HIPAA compliant, this easy-to-use app allows a person’s health status to be authenticated without sharing privacy data.
Under its open system of Certifying Authorities (CA), the QMC HealthID™ App and Platform supports multiple diagnostic tests and unique partner protocols. This provides the flexibility for organizations to set different rules and clearances for screening an Innova “health passport.” As many governments, such as the United Kingdom’s National Health Service, may have their own tracking app, the Innova architecture will adapt to different protocols. A private entity can develop a unique platform.
QMC HealthID™ Incorporated is a wholly-owned subsidiary of Quantum Materials Corp (QMC). Stephen B. Squires, CEO, says, “This ecosystem offers a comprehensive set of COVID-19 testing options that can address the health safety needs of a myriad of industries and testing environments.”
 Using QMC proprietary blockchain technology, an individual’s health information remains private while instant data transmission and authentication to health officials help prevent the virus spread. The smartphone app (Android and iPhone devices) can identify people who have tested negative and aid in safely reopening communities. This always-on service provides end-to-end visibility to support testing and immunization for infectious diseases on a global scale.
Using QMC proprietary blockchain technology, an individual’s health information remains private while instant data transmission and authentication to health officials help prevent the virus spread. The smartphone app (Android and iPhone devices) can identify people who have tested negative and aid in safely reopening communities. This always-on service provides end-to-end visibility to support testing and immunization for infectious diseases on a global scale.
Besides tracking the antigen rapid diagnostic test, QMC HealthID™ signed a distribution agreement with Innova Medical Group Inc. for molecular and antibody tests, as well as PCR swab tests to be included in its COVID-19 Ecosystem. Any person registered on the QMC HealthID™ platform can use an Innova supplied test kit prescribed by a healthcare provider.
***The Innova rapid antigen test is currently not for sale in the USA***